120 rows We observe a common trend in properties as we move across a period from left to right or down the group. For example the format of the periodic table is designed so properties can be easily compared.

What Is Electronegativity Trends Chart Periodic Table Chemtalk
Different types of chemical bonding and patterns and trends can be observed in their arrangement.
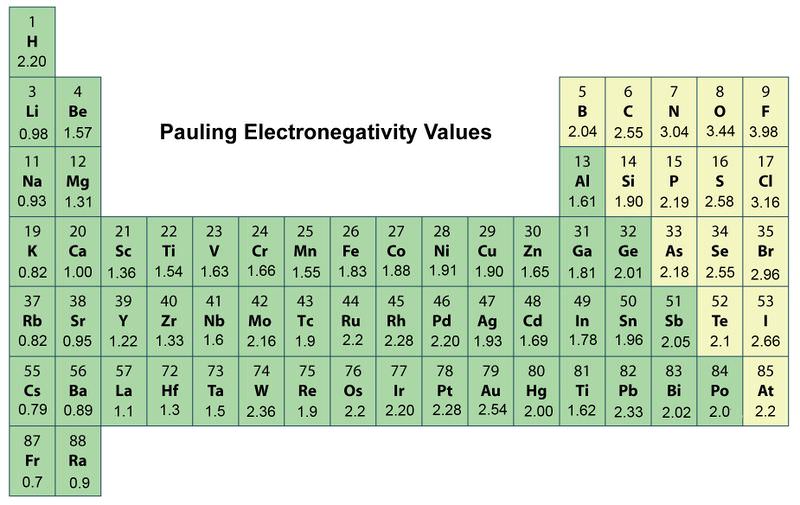
. Trends in electronegativity down a group. In general electronegativity decreases as you move down a group in the periodic table this correlates. When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases.
As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. Since the electronegativity of some of. The answer lies in its atomic structure of course and just like several other properties of elements the periodic table has distinct trends in electronegativity.
The chart shows electronegativities from sodium to chlorine - you have to ignore argon. Each shell comprises at least one subshell named s p d f and g. The periodic table arranges all chemical elements in special ways.
As you go down a group electronegativity decreases. The trends for electronegativity is that the value increases across the periods rows of the periodic table. Group Trends Just to review.
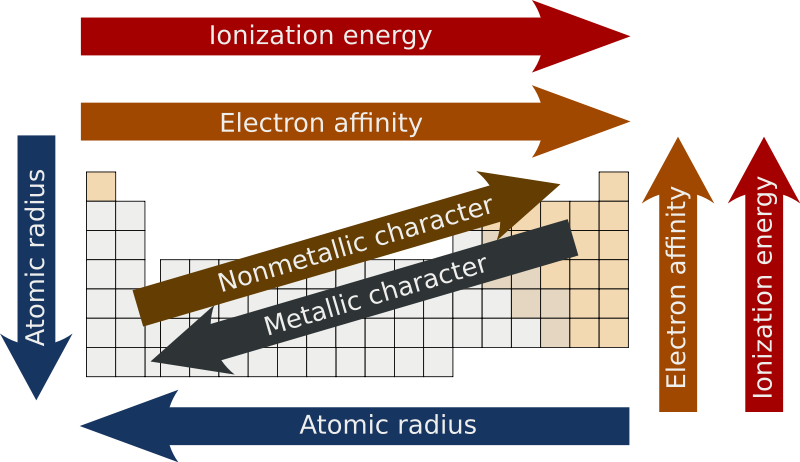
Electronegativity refers to an atoms ability to attract electrons in a chemical bond. A graphic showing overall periodic trends in the periodic table. For example the electronegativity trend across period 3 in the periodic table is depicted below.
The electron setup or organization of electrons circling neutral particles demonstrates a common example of periodicity. As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. Periodic trends are specific patterns in the properties of chemical elements that are revealed in the periodic table of elements.
This scale ranges from 07 to 40 where cesium is the least electronegative element and with fluorine being the most electronegative element. Which of the following trends is similar to electronegativity follows the same pattern. Periodic Trends and Patterns 1.
9th - 12th grade. Periodic Table Trends. This trend in properties is known as periodic properties.
The trend closely resembles electronegativity Increases as you move left to right across a period cokephysicsuclaedulaptag mchselectronegativity-sjpg. As you go across a period the electronegativity increases. Major periodic trends include electronegativity ionization energy electron affinity atomic radii ionic.
What is period trend for electronegativity. Trends in electronegativity across a period. Similarities and Trends in the Periodic Table There are certain trends and patterns in the way elements react and behave.
As you move from left to right across the Periodic Table electronegativity increases and as you move down the table electronegativity decreases. In the groups 6-12 the electronegativity has an increase trend downIn other groups the electronegativity has a decrease trend downIn a period the generally trend is an increase from left to right. The important periodic properties are atomic size metallic character non-metallic character ionization potential electron affinity and electronegativity.
The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. Periodic Trends in the Electronegativities of Elements. 1 on a question What general trend does electronegativity follow on the periodic table.
Lithium 10 and Fluorine 40 in period 2 The electronegativity also increases up a group column of the periodic table. It doesnt have an electronegativity because it doesnt form bonds. There are no units involved nor is it a property but is a relative scale from 1-4.
This is observed through Periodic Trends as the further right of a period and higher up a group are observed to have increased values in electronegativity. Meaning of the Atomic Radius The atomic radius of a chemical element is a measure of the size of its atoms usually the mean or typical distance from the nucleus to the. Lithium 10 and Francium 07 in Group I.
The electrons involve a progression of electron shells numbered 1 2 etc.
What Trend In Electronegativity Do You See As You Go Across A Period Row On The Periodic Table Socratic
What Are 4 Trends That Happen While Going Across The Periodic Table Quora

Electronegativity Definition And Trend
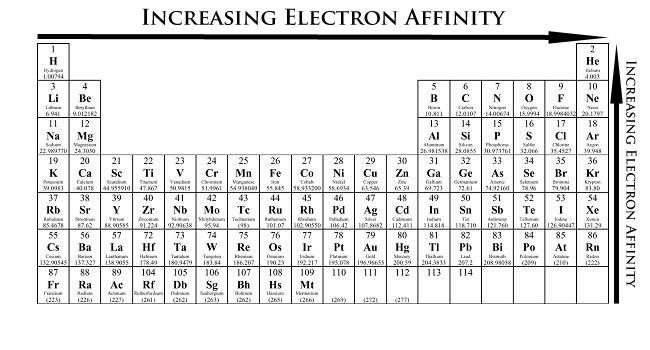
Periodic Trends Chemistry Libretexts

Periodic Trends Made Easy Chemtalk
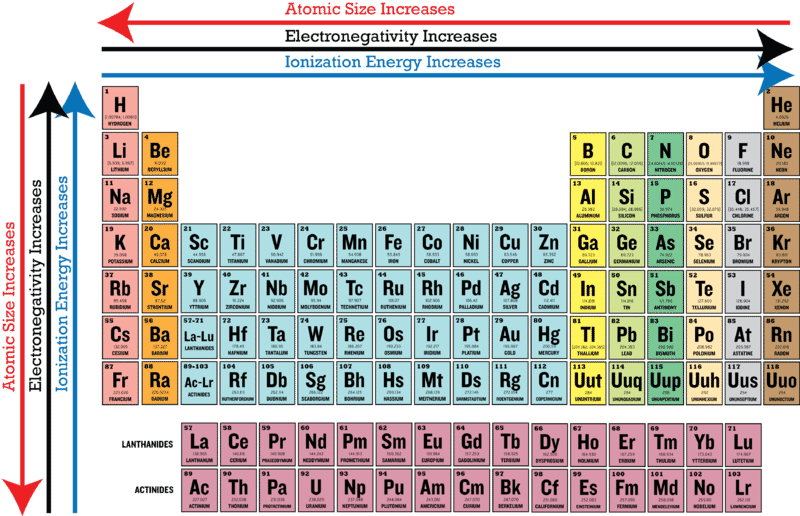
0 comments
Post a Comment